Earth Science Week Classroom Activities
Chemistry of Burning
Activity Source:
The University of Texas at Austin Bureau of Economic Geology. Provided by Association of American State Geologists. Adapted with permission.
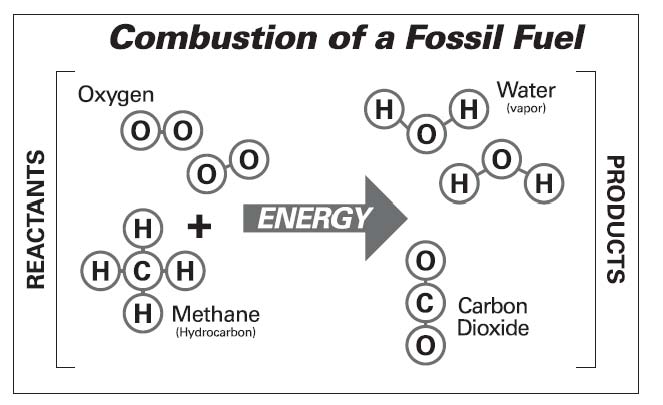
Why is CO2 increasing in the atmosphere? Who is doing it? Many people think that CO2 is “pollution,” so that clean burning should be a way to eliminate greenhouse gas emissions. In this demonstration, we review basic chemistry (see illustration) to realize that producing CO2 is an inevitable product of burning any fossil fuel.
Materials
- At least three large-size plastic foam balls colored white, representing oxygen
- At least one medium-size plastic foam ball colored red, representing carbon
- At least four small-size plastic foam balls colored blue, representing hydrogen
- Pipe cleaners
- Safety scissors
Procedure
1. Prepare plastic foam balls of various sizes and colors (see above). Use safety scissors to cut several pipe cleaners into 1 inch lengths.
Discuss: What is in a hydrocarbon? A hydrocarbon is made up of hydrogen and carbon. For example, one carbon molecule attached to four hydrogen molecules is methane, the simplest of the hydrocarbons.
2. Make a model of a hydrocarbon molecule by linking the appropriate hydrogen and carbon balls with short pieces of pipe cleaner.
Discuss: How do we get energy from hydrocarbon? A hydrocarbon produces energy when it burns, which means adding oxygen to the fuel in the presence of threshold heat.
3. Use pipe cleaners to add oxygen balls, and pull hydrogen balls off the methane. (You can say “pop” or “bang” as you do so, to symbolize the release of energy.) Add two hydrogen balls to each oxygen ball, and add two oxygen balls to each carbon ball to complete modeling these chemical reactions.
Discuss: What are the products of the combustion of fossil fuel? Consider that CO2 is carbon dioxide and H2O is water. Gently toss the model molecules in the air to emphasize what happens to them under “business as usual.”
Discuss: What do we usually see coming out of the tailpipes of cars or from smokestacks on a cool morning? This white “smoke” is water vapor condensing. People often are surprised that combustion releases water. We cannot see the CO2, but there is at least half as much CO2 produced as water from most kinds of combustion.
The University of Texas at Austin Bureau of Economic Geology. Provided by Association of American State Geologists.
Models made of plastic foam balls are used to illustrate the chemistry of combustion.
